Basal Cell Neoplasms Clinical Trial Pipeline Accelerates as 22+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Basal Cell Neoplasms are the most common type of skin cancer, arising from basal cells in the epidermis and typically characterized by slow growth and low metastatic potential. The increasing prevalence of skin cancers due to rising UV exposure and an aging population is driving demand for effective diagnostics and treatment options for basal cell neoplasms. Additionally, the launch of therapies such as Patidegib, AIV001, FLD-103, VP-315, Silmitasertib, RP1, SP-002, R 5780, STP705, BO-112, AVX001, and others will further drive the market.
New York, USA, Sept. 10, 2025 (GLOBE NEWSWIRE) -- Basal Cell Neoplasms Clinical Trial Pipeline Accelerates as 22+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Basal Cell Neoplasms are the most common type of skin cancer, arising from basal cells in the epidermis and typically characterized by slow growth and low metastatic potential. The increasing prevalence of skin cancers due to rising UV exposure and an aging population is driving demand for effective diagnostics and treatment options for basal cell neoplasms. Additionally, the launch of therapies such as Patidegib, AIV001, FLD-103, VP-315, Silmitasertib, RP1, SP-002, R 5780, STP705, BO-112, AVX001, and others will further drive the market.
DelveInsight’s 'Basal Cell Neoplasms Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for basal cell neoplasms across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the basal cell neoplasms domain.
Key Takeaways from the Basal Cell Neoplasms Pipeline Report
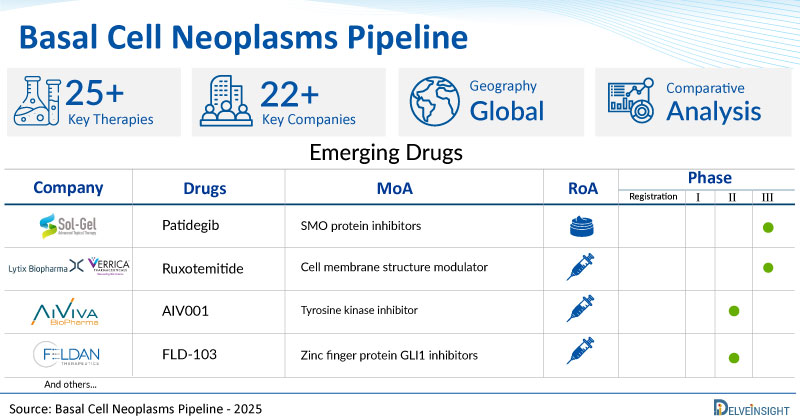
- DelveInsight’s basal cell neoplasms pipeline report depicts a robust space with 22+ active players working to develop 25+ pipeline basal cell neoplasms drugs.
- Key basal cell neoplasms companies such as Sol-Gel Technologies, AiViva BioPharma, Inc., Feldan Therapeutics, Verrica Pharmaceuticals Inc., Senhwa Biosciences, Replimune Inc., Stamford Pharmaceuticals, Inc., Rise Therapeutics, Sirnaomics, Highlight Therapeutics, Coegin Pharma AB, and others are evaluating new basal cell neoplasms drugs to improve the treatment landscape.
- Promising pipeline basal cell neoplasms therapies, such as Patidegib, AIV001, FLD-103, VP-315, Silmitasertib, RP1, SP-002, R 5780, STP705, BO-112, AVX001, and others, are in different phases of basal cell neoplasms clinical trials.
- Promising MoAs in clinical trial include SMO protein inhibitors, Tyrosine kinase inhibitors, Phospholipase A2 modulators, Zinc finger protein GLI1 inhibitors, Cell membrane structure modulator, Casein kinase II inhibitors, and others.
- In April 2025, Senhwa Biosciences, Inc. announced the completion of Clinical Study Report (CSR) for Phase I/Dose Expansion Trial of Silmitasertib (CX-4945) in the Treatment of Basal Cell Carcinoma(BCC), with positive data outcomes. CX-4945 significantly prolonged survival in advanced cancer patients, marking a major milestone for both the patients and Senhwa.
- In April 2025, Zynext Ventures, the venture capital arm of Zydus Lifesciences (Zydus) announced its investment in Feldan Therapeutics for the development of treatments based on intracellular delivery of therapeutics. The company's lead candidate, FLD-103, is administered directly into basal cell carcinoma lesions, where the Shuttle peptide facilitates the delivery of a Hedgehog inhibitor to its target within BCC cells.
- In January 2025, Rise Therapeutics announced that the US Food and Drug Administration has accepted its investigational new drug application to proceed with a cancer Phase I clinical trial for its program candidate, R-5780. Next steps will see to the beginning of the multi-dose Phase I RISE R-5780-01 trial (NCT06398418) evaluating the safety and tolerability of R-5780 in patients currently receiving PD-1 pathway checkpoint inhibitors in patients with melanoma, basal cell carcinoma, or squamous cell carcinoma.
- In January 2025, Stamford Pharmaceuticals announced positive results from Phase II Study of SP-002 in combination with 4-weeks of vismodegib in multi-lesional Basal Cell Carcinoma patients.
- In January 2025, Verrica Pharmaceuticals Inc. announced positive topline findings from part 2 of its Phase II study on VP-315 at the 2025 Winter Clinical Miami Dermatology Conference, held January 17-19, 2025, in Miami, Florida.
Request a sample and discover the recent advances in basal cell neoplasms drugs @ Basal Cell Neoplasms Pipeline Report
The basal cell neoplasms pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage basal cell neoplasms drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the basal cell neoplasms clinical trial landscape.
Find out more about basal cell neoplasms drugs @ Basal Cell Neoplasms Treatment
Basal Cell Neoplasms Competitive Landscape
Leading companies such as Sol-Gel Technologies, AiViva BioPharma, Inc., Feldan Therapeutics, Verrica Pharmaceuticals Inc., Senhwa Biosciences, Replimune Inc., Stamford Pharmaceuticals, Inc., Rise Therapeutics, Sirnaomics, Highlight Therapeutics, Coegin Pharma AB, and others are currently active in the basal cell neoplasms competitive landscape.
Among these, Sol-Gel Technologies (Patidegib) and Lytix Biopharma/Verrica Pharmaceuticals (Ruxotemitide) are evaluating their drugs in the late stage of development. Patidegib is an experimental small-molecule therapy that targets the hedgehog signaling pathway. Developed as a topical formulation, it aims to reduce tumor burden in patients with Gorlin Syndrome, Basal Cell Carcinomas (BCCs), and potentially other conditions. This first-in-class topical gel, derived from a proprietary hedgehog inhibitor licensed from Infinity Pharmaceuticals, contains patidegib as its active ingredient in SGT-610. By blocking the SMO signal, patidegib helps restore normal cellular function and decreases the formation of new tumors.
Whereas, the drugs in the early stage of development include AIV001 (AiViva BioPharma, Inc.), FLD-103 (Feldan Therapeutics), Silmitasertib (Senhwa Biosciences), AVX001 (Coegin Pharma AB), and others.
Learn more about the emerging basal cell neoplasms therapies @ Basal Cell Neoplasms Clinical Trials
Basal Cell Neoplasms Therapeutics Assessment
The basal cell neoplasms pipeline report proffers an integral view of the emerging basal cell neoplasms therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Basal Cell Neoplasms Pipeline Report
- Coverage: Global
- Basal Cell Neoplasms Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Basal Cell Neoplasms Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Basal Cell Neoplasms Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Basal Cell Neoplasms Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Basal Cell Neoplasms Therapeutics Assessment By Mechanism of Action: SMO protein inhibitors, Tyrosine kinase inhibitor, Phospholipase A2 modulators, Zinc finger protein GLI1 inhibitors, Cell membrane structure modulator, Casein kinase II inhibitors.
- Key Basal Cell Neoplasms Companies: Sol-Gel Technologies, AiViva BioPharma, Inc., Feldan Therapeutics, Verrica Pharmaceuticals Inc., Senhwa Biosciences, Replimune Inc., Stamford Pharmaceuticals, Inc., Rise Therapeutics, Sirnaomics, Highlight Therapeutics, Coegin Pharma AB, and others.
- Key Basal Cell Neoplasms Pipeline Therapies: Patidegib, AIV001, FLD-103, VP-315, Silmitasertib, RP1, SP-002, R 5780, STP705, BO-112, AVX001, and others.
Dive deep into rich insights for new basal cell neoplasms treatments, visit @ Basal Cell Neoplasms Drugs
Table of Contents
| 1. | Basal Cell Neoplasms Pipeline Report Introduction |
| 2. | Basal Cell Neoplasms Pipeline Report Executive Summary |
| 3. | Basal Cell Neoplasms Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Basal Cell Neoplasms Clinical Trial Therapeutics |
| 6. | Basal Cell Neoplasms Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Basal Cell Neoplasms Pipeline: Late-Stage Products (Phase III) |
| 8. | Basal Cell Neoplasms Pipeline: Mid-Stage Products (Phase II) |
| 9. | Basal Cell Neoplasms Pipeline: Early-Stage Products (Phase I) |
| 10. | Basal Cell Neoplasms Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Basal Cell Neoplasms Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Basal Cell Neoplasms Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the basal cell neoplasms pipeline therapeutics, reach out @ Basal Cell Neoplasms Therapeutics
Related Reports
Basal Cell Neoplasms Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key basal cell neoplasms companies, including Sol-Gel Technologies, AiViva BioPharma, Inc., Feldan Therapeutics, Incyte Corporation, Verrica Pharmaceuticals Inc., Senhwa Biosciences, Turn Therapeutics, Shanghai Fudan-Zhangjiang Bio-Pharmaceutical, Palvella Therapeutics, TransDerm, among others.
Basal Cell Carcinoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key basal cell carcinoma companies, including MedC Biopharma Corporation, AiViva BioPharma, MediWound, Kintara Therapeutics, IO Biotech, Sirnaomics, Aresus Pharma, Epitome Pharmaceuticals, Transgene, Senhwa Biosciences, Palvella Therapeutics, Suzhou Kintor Pharmaceuticals, Leaf Vertical, among others.
Basal Cell Carcinoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key basal cell carcinoma companies, including PellePharm, MedC Biopharma Corporation, AiViva BioPharma, MediWound, Kintara Therapeutics, IO Biotech, Sirnaomics, Aresus Pharma, Epitome Pharmaceuticals, Transgene, Senhwa Biosciences, Palvella Therapeutics, Suzhou Kintor Pharmaceuticals, Leaf Vertical, among others.
Gorlin Syndrome Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Gorlin syndrome companies, including Palvella Therapeutics, Inc., PellePharm, Ascend Biopharmaceuticals, among others.
Gorlin Syndrome Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Gorlin syndrome companies, including Palvella Therapeutics, Inc., PellePharm, Ascend Biopharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
